Specifications
|
Assay Points
|
500/10000 |
|
Assay Target Type
|
Kit
|
|
Assay Technology
|
HTRF
|
|
Brand
|
HTRF
|
|
Quantity
|
1 |
|
Shipping Conditions
|
Shipped in Dry Ice
|
|
Therapeutic Area
|
Oncology & Inflammation
|
|
Unit Size
|
500/10000 Assay Points
|
Assay principle
Cell supernatant, sample, or standard is dispensed directly into the assay plate for the detection by HTRF® reagents (384-well low-volume white plate or Cisbio low-volume 96-well plate in 20 µl). The antibodies labeled with the HTRF donor and acceptor are pre-mixed and added in a single dispensing step, to further streamline the assay procedure. The assay can be run up to a 1536-well format by simply resizing each addition volume proportionally.

Assay data analysis
The 4 Parameter Logistic (4PL) curve is commonly recommended for fitting an ELISA standard curve. This regression enables the accurate measurement of an unknown sample across a wider range of concentrations than linear analysis, making it ideally suited to the analysis of biological systems like cytokine releases.
Assay details
Technical specifications of human IFN gamma kit
| Sample size |
16 µL |
| Final assay volume |
20 µL |
| Kit components |
Lyophilized standard, frozen detection antibodies, buffers &protocol. |
| LOD &LOQ (in Diluent) |
14 pg/mL &21 pg/mL |
| Range |
21 – 4,000 pg/mL |
| Time to result |
Overnight at RT |
| Calibration |
NIBSC (82/587) value (IU/mL) = 0,019 x HTRF hIFN? value (pg/mL) |
| Species |
Human only |
Analytical performance
Intra and inter assay
Intra-assay (n=24)
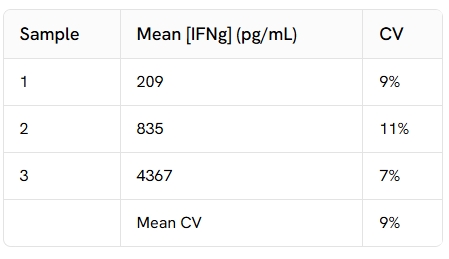
Each of the 3 samples was measured 24 times, and % CV was calculated for each sample.
Inter-assay (n=4)
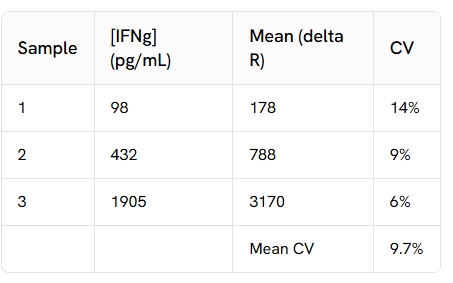
Each of the samples was measured in 4 different experiments, and % CV was calculated for each sample.
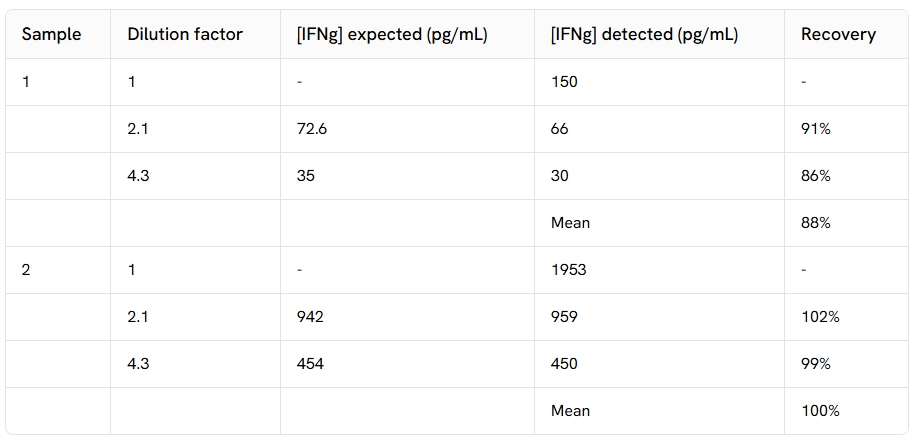
Dilutional linearity
The excellent % of recovery obtained from these experiments show the good linearity of the assay.
Spike and recovery
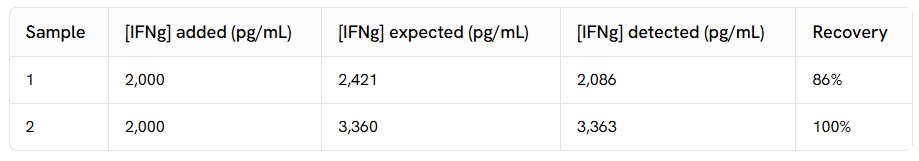
The same amount of recombinant cytokine was added to 2 different serum samples, and the set of responses obtained from a standard curve was compared to the calculated expected values. The ~ 100% of recovery observed validates the sample matrix used for this assay.
Assay validation
IFNg secretion in PBMCs stimulated with PMA and Ionomycin
PBMC plated at 50, 100, 200 and 400 kcells/well were stimulated for 3h with increasing concentrations of PMA (0, 1, 50 ng/mL) added to a 500 ng/mL Ionomycin solution. 16 µL of supernatants were then transferred into a white detection plate (384, low volume) to be analyzed with the Human IFN? Assay.